Categories
Change Password!
Reset Password!


In healthy people, fexuprazan is safe and can be used without consideration of ethnicity.
A randomized, placebo-controlled, double-blind, single- and multiple-dose study published in Alimentary Pharmacology & Therapeutics found gastric acid suppression by fexuprazan (DWP14012, a novel potassium-competitive acid blocker) to be similar among the three ethnicities (Korean, Caucasian and Japanese). Furthermore, the pharmacokinetics, pharmacokinetics- pharmacodynamics relationships and safety were also similar among the ethnicities.
Fexuprazan is under development to manage acid-related disorders. A study was carried out for comparing the pharmacodynamics, pharmacokinetics and safety of fexuprazan in healthy people of Japanese, Caucasian, and Korean descent. A total of 10 participants in each arm (20, 40 or 80 mg for Japanese volunteers; 40 or 80 mg for Caucasian volunteers; 40, 60 or 80 mg for Korean volunteers) were randomized to get either placebo or fexuprazan.
For PK/PD assessment, collection of 24-hour intragastric pH estimations and serial blood samples was done. A comparison of PK/PD parameters between each ethnicity was done. In this trial, the extent of gastric acid inhibition was noted to be comparable among the 3 ethnicities. Table 1 shows mean percentage of time that the intragastric pH was above four following multiple doses of 40 mg and 80 mg fexuprazan in people with different ethnicities.
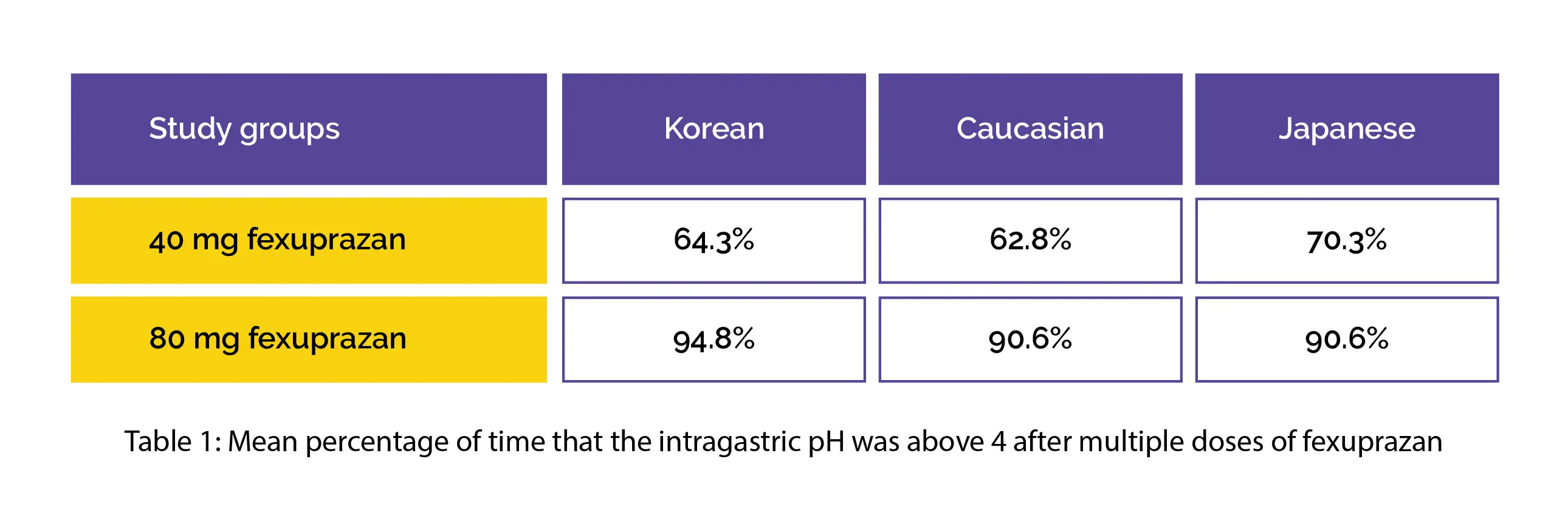
No profound alteration was noted in serum gastrin between the 3 ethnicities. The systemic exposure of fexuprazan was comparable between the 3 ethnicities following 40 mg doses but slightly lower in Japanese and Caucasian volunteers following 80 mg doses. The inhibition of gastric acid by fexuprazan revealed a clear exposure-response relationship in Japanese, Korean, and Caucasian people.
Alimentary Pharmacology & Therapeutics
Pharmacodynamics and pharmacokinetics of DWP14012 (fexuprazan) in healthy subjects with different ethnicities
Jun Gi Hwang et al.
Comments (0)