Categories
Change Password!
Reset Password!


Abrocitinib is safe and effective for the management of atopic dermatitis patients after switching from dupilumab.
The effectiveness and safety profile of oral abrocitinib in JADE EXTEND supported the role of abrocitinib as a promising therapy for people having moderate-to-severe atopic dermatitis, irrespective of previous response status to dupilumab, as elucidated from a phase III study published in the Journal of the American Academy of Dermatology. This study aimed to investigate safety and effectiveness of abrocitinib in atopic dermatitis people who were previously administered dupilumab.
Participants were given 100 mg or 200 mg abrocitinib once-daily in JADE EXTEND (phase III extension) after dupilumab in double-blind, placebo-controlled phase 3 JADE COMPARE. Among previous dupilumab responders and non-responders, ≥75% improvement in Eczema Area and Severity Index (EASI-75) and ≥4-point improvement in Peak Pruritus Numerical Rating Scale (PP-NRS4) was attained in a higher percentage of people who were given 12 weeks of 200 mg abrocitinib when compared to 100 mg abrocitinib, as shown in Table 1:
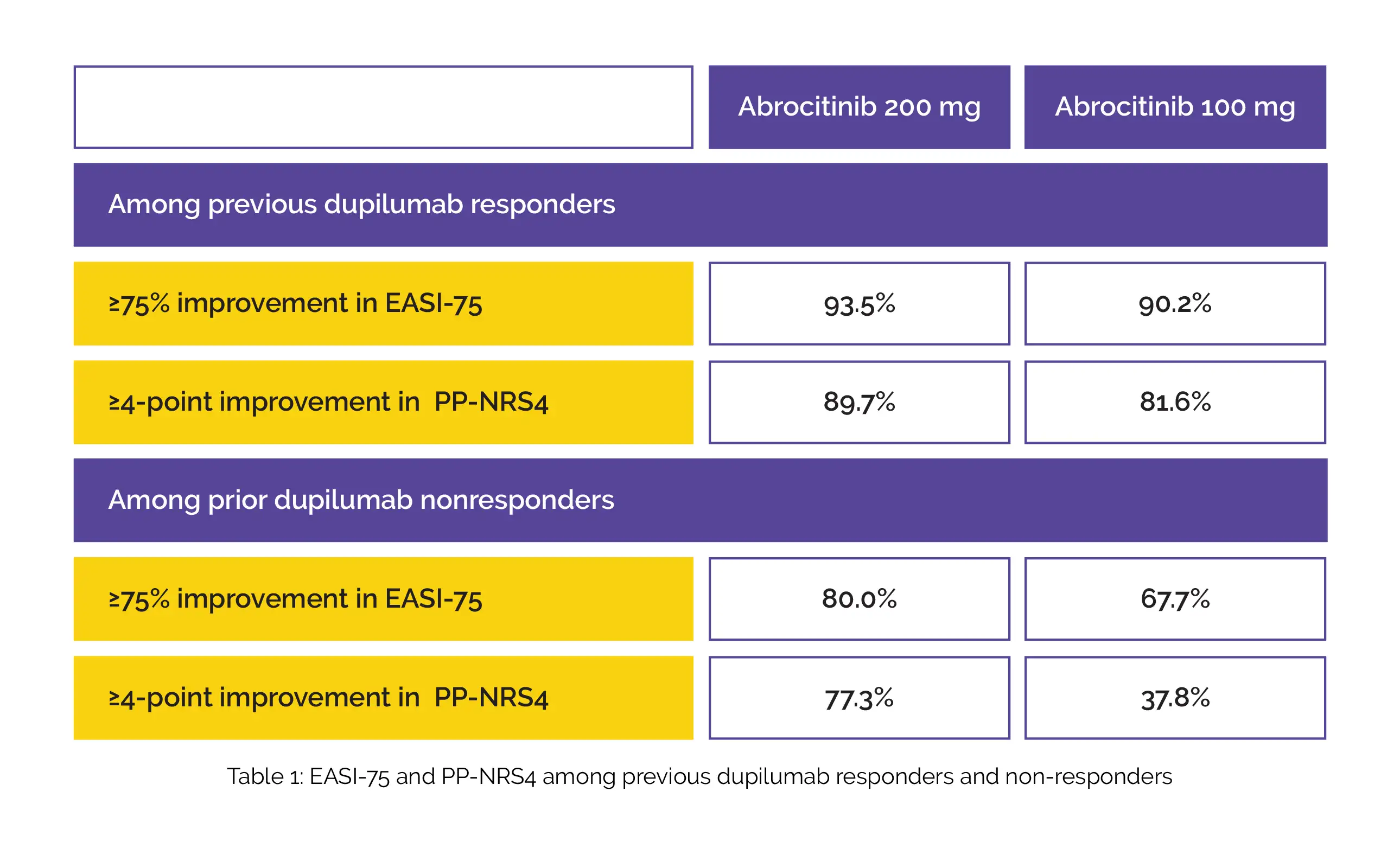
Headache, acne, nausea, and nasopharyngitis were the most common adverse events among abrocitinib-treated people. Conjunctivitis was found to occur less frequently with abrocitinib when compared to previous dupilumab. Hence, adults who responded to previous dupilumab therapy in JADE COMPARE study maintained most of the clinical benefits with abrocitinib therapy for 12 weeks in JADE EXTEND study.
Journal of the American Academy of Dermatology
Phase 3 Efficacy and Safety of Abrocitinib in Adults with Moderate-to-Severe Atopic Dermatitis After Switching from Dupilumab (JADE EXTEND)
Vivian Y. Shi et al.
Comments (0)