Categories
Change Password!
Reset Password!


Tirzepatide notably improves liver condition and fibrosis outcomes, and warrants consideration for inclusion in treatment strategies.
In a recent study that was published in 'The New England Journal of Medicine', Tirzepatide showed noteworthy positive outcomes for individuals with metabolic dysfunction-associated steatohepatitis (MASH, earlier known as nonalcoholic steatohepatitis) and moderate to severe hepatic fibrosis. Over a period of 52 weeks, participants treated with Tirzepatide experienced greater resolution of MASH without an increase in fibrosis than the placebo.
Tirzepatide, a dual agonist targeting insulinotropic polypeptide and glucagon-like peptide-1 receptors, was tested in a double-blind, randomized trial across multiple centres for MASH.
Overall, 157 people with MASH (diagnosed by biopsy) and moderate or severe fibrosis of the total 190 study participants were administered subcutaneous doses of Tirzepatide 5 mg, Tirzepatide 10 mg, or Tirzepatide 15 mg or a placebo every week. Results exposed that Tirzepatide was notably more effective than placebo. Resolution of MASH without fibrosis worsening occurred in only 10% of placebo recipients, compared to 44%, 56%, and 62% in the 5 mg, 10 mg, and 15 mg Tirzepatide groups, respectively (Figure 1):
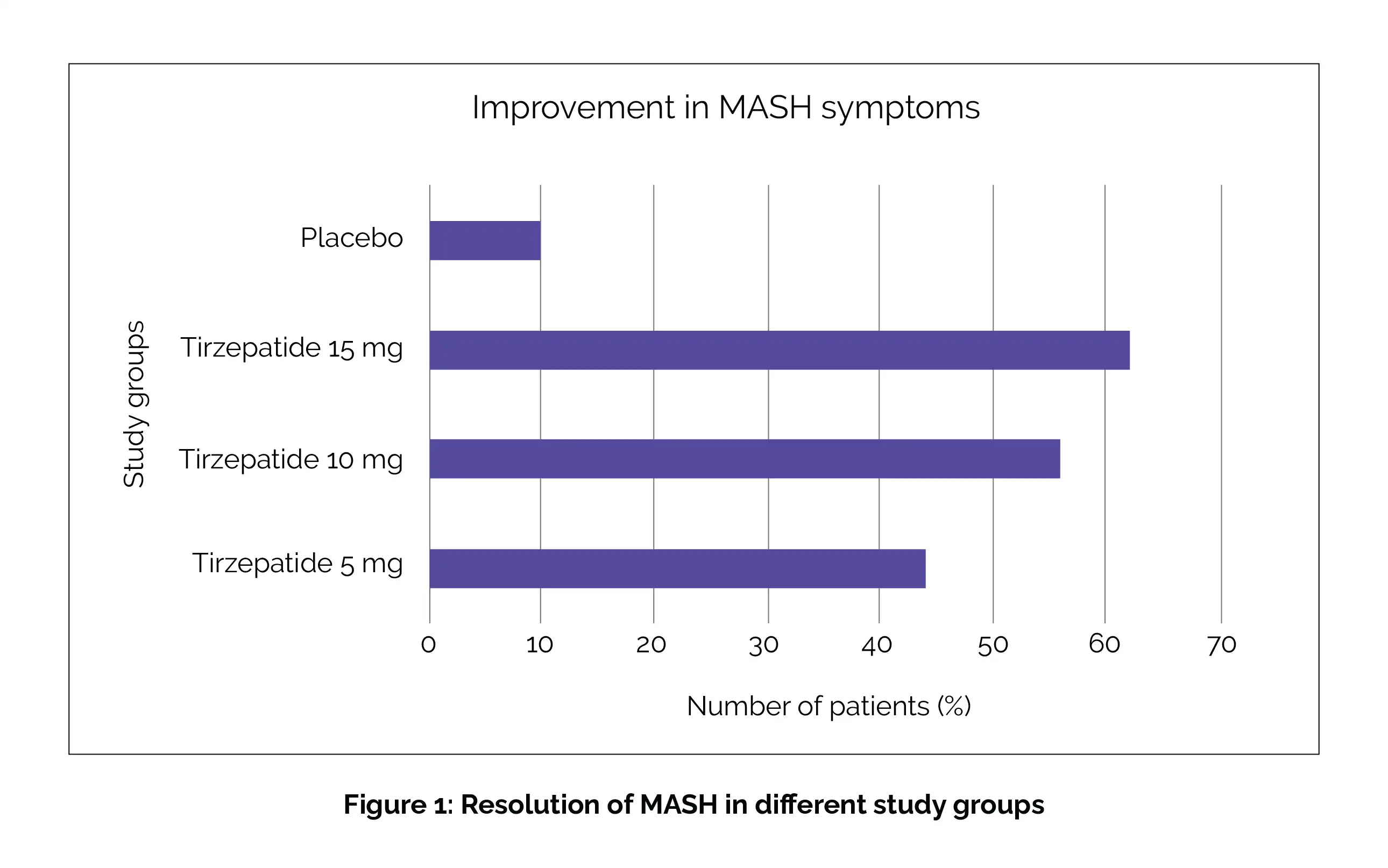
Greater improvement was observed in the fibrosis stage among those receiving Tirzepatide, with rates of 55%, 51% and 51% in the respective dosage groups versus 30% in those with placebo.
Though Tirzepatide showed promise, with mild to moderate gastrointestinal issues being the most common side effects, further extensive studies are needed to fully confirm its efficacy and safety for treating MASH, concluded the study researchers.
The New England Journal of Medicine
Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis with Liver Fibrosis
Rohit Loomba et al.
Comments (0)