Categories
Change Password!
Reset Password!


In patients with moderate-to-severe chronic hand eczema, twice-daily application of topical Delgocitinib is effective and well-tolerated.
A recent phase 3 study, DELTA 1 and DELTA 2, investigated topical Delgocitinib cream (20 mg/g) for moderate-to-severe chronic hand dermatitis management. The trials, conducted across multiple countries, involved 960 adults, who were randomly assigned (2:1) to receive either Delgocitinib cream or a vehicle cream twice daily for 16 weeks.
Clinical success based on the Investigator's Global Assessment for Chronic Hand Eczema (IGA-CHE) at week 16 was the key outcome ascertained. The results demonstrated that a substantially higher proportion of Delgocitinib-treated patients in DELTA 1 and 2 attained clear or almost clear skin when compared to vehicle cream groups. Safety data indicated that adverse events were similar across both groups, with COVID-19 and nasopharyngitis being the most common occurrences (Table 1).
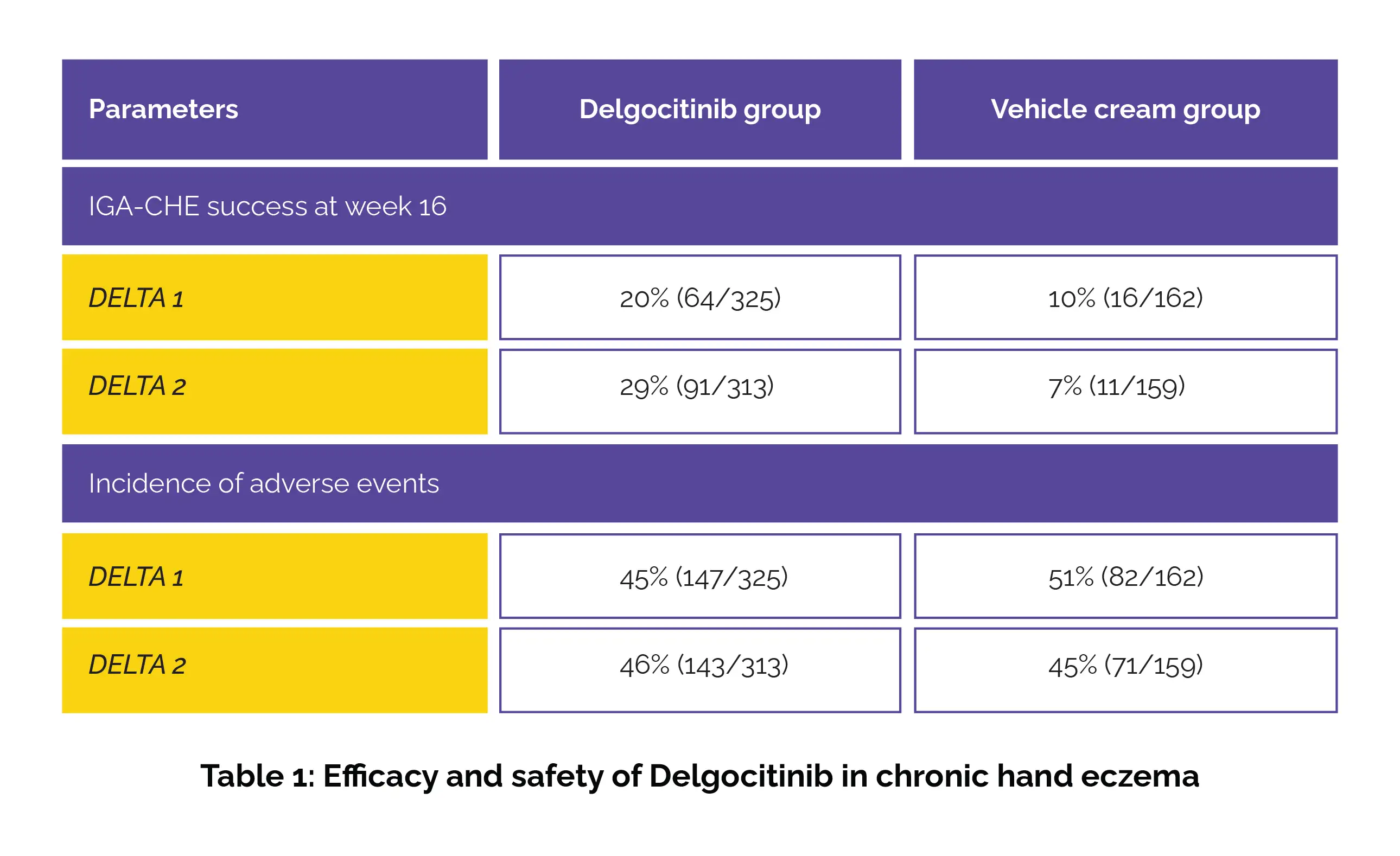
Overall, Delgocitinib (pan-Janus kinase inhibitor) cream was well-tolerated and demonstrated better efficiency than the vehicle cream over the 16-week period. These findings highlight its potential as a valuable option for chronic hand eczema sufferers, particularly those who struggle to manage their condition with standard skincare routines and topical corticosteroids.
The Lancet
Efficacy and safety of delgocitinib cream in adults with moderate to severe chronic hand eczema (DELTA 1 and DELTA 2): results from multicentre, randomised, controlled, double-blind, phase 3 trials
Robert Bissonnette et al.
Comments (0)