Змінити пароль!
Скинути пароль!


Дегенеративні захворювання поперекового відділу хребта (такі як стеноз хребетного каналу та грижа міжхребетного диска) часто викликають сильну компресію корінців поперекових нервів, що призводить до розвитку сильного болю.
Кеторолак, що застосовується у вигляді монотерапії або в поєднанні з іншими знеболюючими препаратами, значно знижує інтенсивність післяопераційного болю та обсяг використання опіоїдів після хірургічної операції на поперековому відділі хребта без суттєвого посилення нудоти чи блювання. Однак його вплив на тривалість госпіталізації незначний.
Дегенеративні захворювання поперекового відділу хребта (такі як стеноз хребетного каналу та грижа міжхребетного диска) часто викликають сильну компресію корінців поперекових нервів, що призводить до розвитку сильного болю. За наявності серйозних симптомів іноді потрібні хірургічні втручання (наприклад, спондилодез та мінімально інвазивна декомпресія). Однак вони пов'язані з післяопераційними проблемами, включаючи біль у спині та біль по ходу уражених нервових корінців. Такий біль може виникнути внаслідок ішемії м'язів, їх розсічення чи пошкодження гілок спинномозкових нервів під час хірургічного втручання. Неналежне лікування болю може ускладнити рухливість пацієнта у ранньому післяопераційному періоді, а також його відновлення. Воно також може підвищити ймовірність виникнення таких ускладнень, як синдром хронічного болю у спині та тромбоз вен нижніх кінцівок.
Сучасні підходи до лікування післяопераційного болю часто ґрунтуються на застосуванні опіоїдів, які забезпечують ефективне полегшення болю. Однак їх використання пов'язане з побічними ефектами, включаючи нудоту, блювання та ризик розвитку стійкої залежності. Надмірне застосування опіоїдів — зростаюча проблема для охорони здоров'я. Результати досліджень вказують на те, що застосування опіоїдів щодо гострого післяопераційного дистресу може через необачність призвести до тривалої залежності. Тому для прискорення післяопераційного відновлення доцільно оптимізувати контроль болю, мінімізуючи при цьому застосування опіоїдів.
Кеторолак - нестероїдний протизапальний препарат (НПЗП), що має опіоїдзберігаючий ефект, - широко застосовують для полегшення післяопераційного болю. Його застосування як монотерапії та в поєднанні з іншими знеболюючими препаратами, такими як бупівакаїн, морфін і парацетамол, вивчали при хірургічних втручаннях на поперековому відділі хребта в численних рандомізованих контрольованих дослідженнях (РКД). Відмінності у дизайні досліджень, демографічних характеристиках пацієнтів та режимах застосування препарату призвели до різних результатів, що ускладнює розробку стандартизованих протоколів лікування болю.
Обґрунтування дослідження
Відсутність чітких клінічних рекомендацій щодо застосування кеторолаку в комбінованій терапії болю наголошує на необхідності проведення комплексного аналізу існуючих даних.
Ціль
Мета даного систематичного огляду та метааналізу полягала в оцінці ефективності та безпеки застосування кеторолаку при хірургічних втручаннях на поперековому відділі хребта, спрямованої на отримання науково обґрунтованих даних для оптимізації контролю болю, зниження залежності від опіоїдів та покращення результатів лікування пацієнтів.
Пошук літератури
Здійснено систематичний пошук у базах даних, включаючи Кокрейнівську бібліотеку, China National Knowledge Infrastructure (CNKI), Elton B. Stephens Company (EBSCO), Embase, Medline, PubMed, VIP, WanFang та Web of Science, з моменту їх створення до травня 2023 року. При пошуку використовували ключові слова та терміни медичних предметних рубрик (MeSH), такі як "анальгезія", "кеторолак", "хірургічна операція на поперековому відділі хребта", "хірургічна операція на хребті" та "післяопераційний біль". Списки літератури відповідних досліджень та оглядів також переглядали вручну та повторювали процес для виявлення додаткових відповідних критеріїв досліджень. У липні 2024 р. було виконано остаточний пошук, результати якого підтвердили, що до огляду були включені найбільш актуальні дані на момент оцінки.
Критерії включення
Критерії виключення
Вилучення даних
З оригінальних досліджень витягували вихідну інформацію для обох груп, включаючи розмір вибірки, ПІБ провідного автора, країну, вік, стать, введення препарату (метод та час), хірургічне втручання (тривалість та тип). Збір даних здійснювали двоє дослідників незалежно друг від друга, а розбіжності врегулювали з урахуванням узгодженого рішення. При необхідності отримання додаткових даних, пов'язувалися з автором, відповідальним за кореспонденцію.
Дані та статистичний аналіз
Дослідники аналізували безперервні показники (такі як оцінки за візуальною аналоговою шкалою (ВАШ), тривалість госпіталізації, застосування морфіну у післяопераційний період). При аналізі використовували середню різницю (СР) або стандартну середню різницю (ССР) із зазначенням 95 % довірчих інтервалів (ДІ). ССР використовували для показників, виміряних за різними шкалами. Дихотомічні показники (наприклад, небажані явища) оцінювали з використанням відношення ризиків (ВР) із зазначенням 95 % ДІ.
Щоб врахувати очікувані відмінності в дизайні досліджень, популяціях та втручаннях, було обрано модель з випадковими ефектами, при цьому для аналізу досліджень з однорідними характеристиками використовували модель з фіксованими ефектами. Оцінку статистичної гетерогенності виконували з використанням Q-критерію та статистики I² з пороговими значеннями I² 25, 50 та 75 % (що відповідає низькій, помірній та високій гетерогенності).
При проведенні аналізу у підгрупах вивчали джерела гетерогенності, приділяючи особливу увагу часу застосування кеторолаку (передопераційний, інтраопераційний, післяопераційний період) та його дозі, оскільки ці фактори можуть впливати на його ефективність щодо лікування болю та тривалості госпіталізації.
Оцінка ризику систематичних помилок та оцінка якості
Перевірку на наявність систематичної помилки, пов'язаної з переважною публікацією позитивних результатів досліджень, виконували за допомогою критеріїв Бегга та Еггера, а також лійкоподібної діаграми. Однак через обмежену статистичну потужність критерію дану оцінку не проводили для показників, вивчених менш ніж у 10 дослідженнях. Поріг статистичної значущості було встановлено лише на рівні p < 0,05. Аналіз виконували за допомогою програмного забезпечення OpenMeta-Analyst з використанням компонента R як статистичного движка.
Методологічні стандарти кожного РКД визначали з використанням шкали Джадада. З її допомогою оцінювали рандомізацію (0–2 бали), застосування сліпого методу (0–2 бали) та вибуття пацієнтів із дослідження/припинення участі пацієнтів у дослідженні (0–1 бал). За кожен пункт присуджували 1 бал, якщо він був належним чином виконаний та відображений у документації. Оцінка за шкалою Джадада варіювала від 0 до 5 балів, де оцінка 2 балів означала низьку якість, а оцінка 3 балів — висока якість. Шкала Джадада була обрана через її простоту, ефективність та сфокусованість на ключових елементах, таких як рандомізація та застосування сліпого методу. Незважаючи на більш високу деталізацію інструменту Кокрейновського співробітництва для оцінки ризику систематичної помилки, перевага була віддана шкалі Джадада через властивий їй баланс між строгістю та практичністю.
Кінцеві точки дослідження
Оцінювали такі основні кінцеві точки:
Оцінювали такі додаткові кінцеві точки:
Блок-схема відбору досліджень
На малюнку 1 представлена блок-схема з переважними параметрами звітності для систематичних оглядів та метааналізу (PRISMA), що описує процедуру скринінгу.
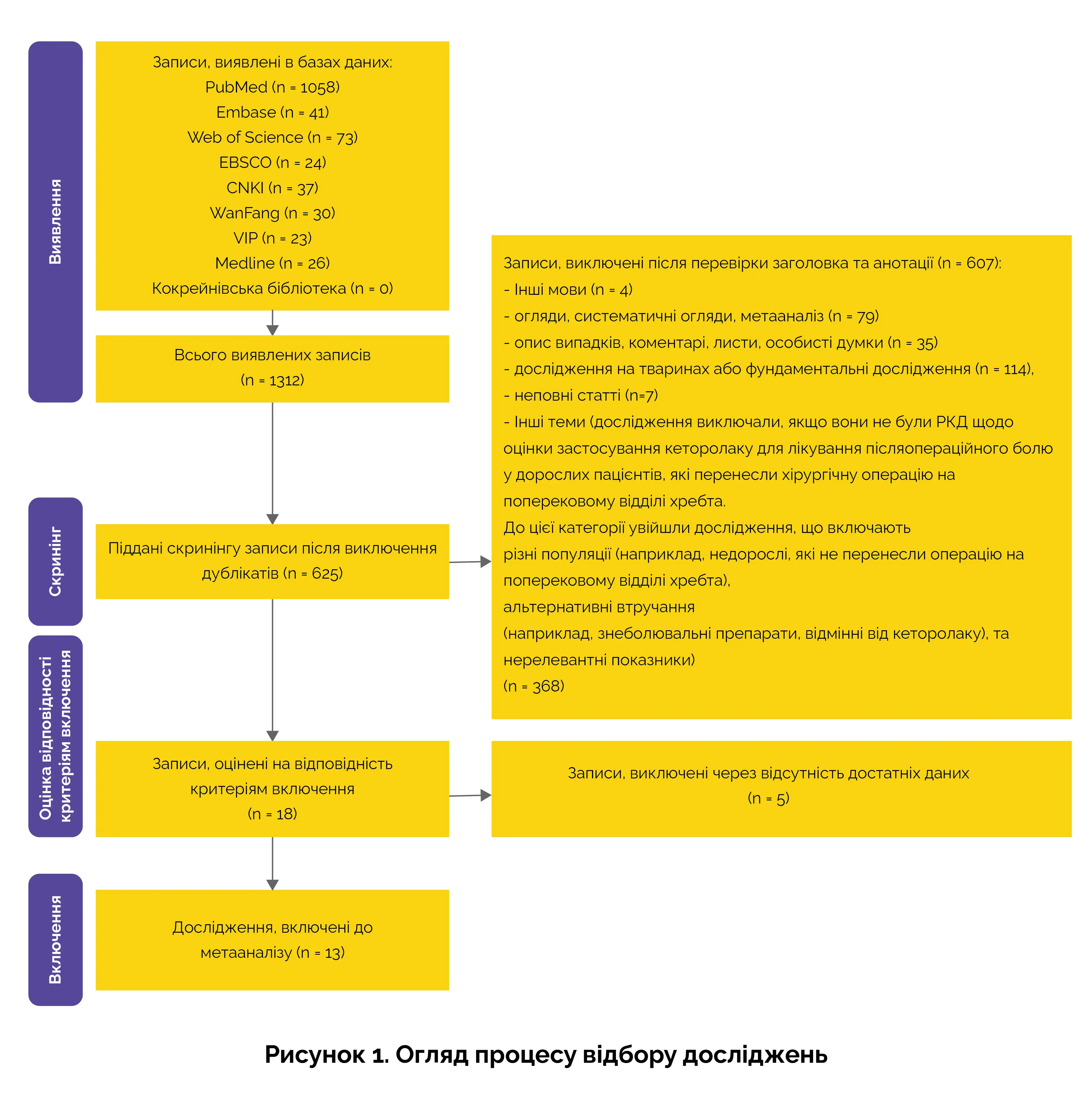
Основні результати
Було включено загалом 13 РКД за участю 938 пацієнтів. Дослідження характеризувались високим рівнем методологічної однаковості. Слід зазначити, що 3 дослідження отримали оцінку 5 балів за шкалою Джадада, 6 досліджень – 4 бали, а 4 дослідження – 3 бали. Основні результати представлені нижче.
(a) 0-6 годин: застосування кеторолаку призводило до істотного зниження інтенсивності болю, при цьому СР склала -1,42 (95% ДІ: від -2,03 до -0,80). Це значення перевищувало мінімальну клінічно значущу різницю (МКЗР) у 1,2–2,0 бала за ВАШ, що вказувало на істотне зниження інтенсивності болю.
(b) 6–12 годин: зниження інтенсивності болю залишалося суттєвим, при цьому СР склала -0,58 (95 % ДІ: від -0,80 до -0,35), проте вона була нижчою за пороговий МКЗР.
(c) 12-24 години: застосування кеторолаку, як і раніше, забезпечувало суттєве полегшення болю, при цьому значення СР склало -0,48 (95 % ДІ: від -0,68 до -0,28). Однак це зниження також виявилося нижчим за пороговий МКЗР.
На закінчення слід зазначити, що результати цього дослідження підтверджують ефективність застосування кеторолаку як знеболювального препарату для усунення післяопераційного болю у пацієнтів, які перенесли хірургічну операцію на поперековому відділі хребта. Згідно з результатами метааналізу 13 РКД за участю 932 пацієнтів, застосування кеторолаку у вигляді монотерапії або у поєднанні з іншими знеболюючими препаратами помітно знижувало оцінки інтенсивності болю протягом перших 12 годин після хірургічного втручання, при цьому найбільше полегшення болю відзначалося протягом перших 6 годин. Зниження інтенсивності болю протягом 6–12 годин та 12–24 годин після втручання було статистично значущим, хоча клінічна значущість останнього зниження виявилася нижчою за МКЗР.
Ключовою знахідкою є опіоїдзберігаючий ефект кеторолаку: його застосування забезпечувало значне зниження потреби в морфіні в післяопераційний період, що є вирішальним фактором для мінімізації побічних ефектів, пов'язаних із застосуванням опіоїдів та залежності від них. Результати дослідження вказували на те, що застосування кеторолаку призводило до значного зменшення залежності від морфіну в післяопераційний період при ССР -1,83, проте спостерігалася істотна гетерогенність результатів. Проведення аналізу чутливості дозволило зменшити цю варіабельність шляхом виключення деяких досліджень з викидами, що призвело до отримання більш узгоджених результатів. Кеторолак був ефективний при різних дозах та різному часі введення (у передопераційний, інтраопераційний та післяопераційний період), що вказує на його універсальність при комбінованих тактиках лікування болю.
Результати дослідження також вказували на те, що застосування кеторолаку пов'язане із меншою тривалістю госпіталізації, зниженням використання лікарняних ресурсів та потенційним зменшенням витрат на охорону здоров'я. Середнє скорочення тривалості госпіталізації становило 0,45 дня. Незважаючи на те, що це скорочення було не суттєвим з погляду клінічного одужання, воно може мати значення для оптимізації ресурсів у хірургічних відділеннях. Незважаючи на більш високі прямі витрати на препарат, пов'язані із застосуванням кеторолаку, його загальна економічна ефективність була підкріплена скороченням використання опіоїдів, що призводило до зниження загальних витрат на госпіталізацію та швидше одужання.
Відповідно до результатів аналізу безпеки, застосування кеторолаку не викликало суттєвого збільшення частоти розвитку поширених післяопераційних небажаних явищ, таких як нудота, блювання, свербіж чи запор, порівняно з іншими знеболюючими препаратами. Однак його застосування все ж таки може провокувати у пацієнтів більш серйозні ускладнення, включаючи гостре пошкодження нирок, шлунково-кишкову кровотечу та анафілаксію, особливо у пацієнтів з вже наявними захворюваннями нирок або факторами ризику з боку шлунково-кишкового тракту. Результати дослідження свідчать про те, що ці ризики можна знизити шляхом моніторингу функції нирок і вжиття таких запобіжних заходів як одночасне застосування інгібіторів протонної помпи у схильних до високого ризику пацієнтів.
Таким чином, кеторолак показав себе як цінний лікарський препарат для контролю післяопераційного болю у пацієнтів, які перенесли хірургічну операцію на поперековому відділі хребта, оскільки він забезпечував клінічно значуще полегшення болю та скорочував залежність від опіоїдів. Однак рішення про його застосування має ґрунтуватися на ретельному аналізі, особливо у пацієнтів з високим ризиком, зважаючи на можливість розвитку серйозних небажаних явищ. Майбутні дослідження повинні розширити наявні дані, що дозволить отримати більш чітке уявлення про профіль безпеки препарату в різних групах пацієнтів та хірургічні ситуації. Це допоможе провести більш точну оцінку співвідношення «ризик – користь» та уточнити клінічні рекомендації щодо безпечного застосування препарату у післяопераційному періоді. Крім того, майбутні дослідження мають бути зосереджені на стандартизації протоколів та розробці ідеальних стратегій застосування препарату.
У дорослих пацієнтів, які перенесли хірургічну операцію на поперековому відділі хребта, застосування кеторолаку виявилося дуже ефективним методом лікування післяопераційного болю та зниження використання опіоїдів, що не викликає підвищення частоти розвитку таких небажаних явищ, як нудота та блювання. Незважаючи на те, що застосування препарату призводило до невеликого скорочення тривалості госпіталізації, клінічний ефект скорочення залишається мінімальним.
Systematic Reviews
The efficacy and safety of ketorolac for postoperative pain management in lumbar spine surgery: a meta-analysis of randomized controlled trials
Jianbin Guan та співавт.
Коментарі (0)