Categories
Change Password!
Reset Password!


An intra-articular Triamcinolone injection and a combination of intra- and extraarticular Dextrose prolotherapy were examined to determine the therapeutic effects on osteoarthritis of the knee in a randomized clinical trial.
People with knee osteoarthritis benefit both in the short and midterm from a combination of intra-articular and neurofascial Dextrose prolotherapy.
An intra-articular Triamcinolone injection and a combination of intra- and extraarticular Dextrose prolotherapy were examined to determine the therapeutic effects on osteoarthritis of the knee in a randomized clinical trial.
Overall, 50 knee osteoarthritis subjects were segregated into 2 groups. The 1st group got Dextrose prolotherapy in the form of 1 intra-articular injection of 10cc 16% Dextrose and periarticular intradermal injections of 12% Dextrose at four sites surrounding the knee (2.5 cc at each position). Triamcinolone (40 mg) was injected intra-articularly for the second group.
Both therapies significantly reduced pain (assessed by visual analog scale [VAS]) and Western Ontario and McMaster Universities Arthritis (WOMAC) (all of its components) at 1 and 3 months post-intervention compared to pre-treatment. The corticosteroid group exhibited superior pain reduction in the first month. The prolotherapy group showed substantially higher improvements in the VAS and WOMAC components in the third post-intervention month (Table 1).
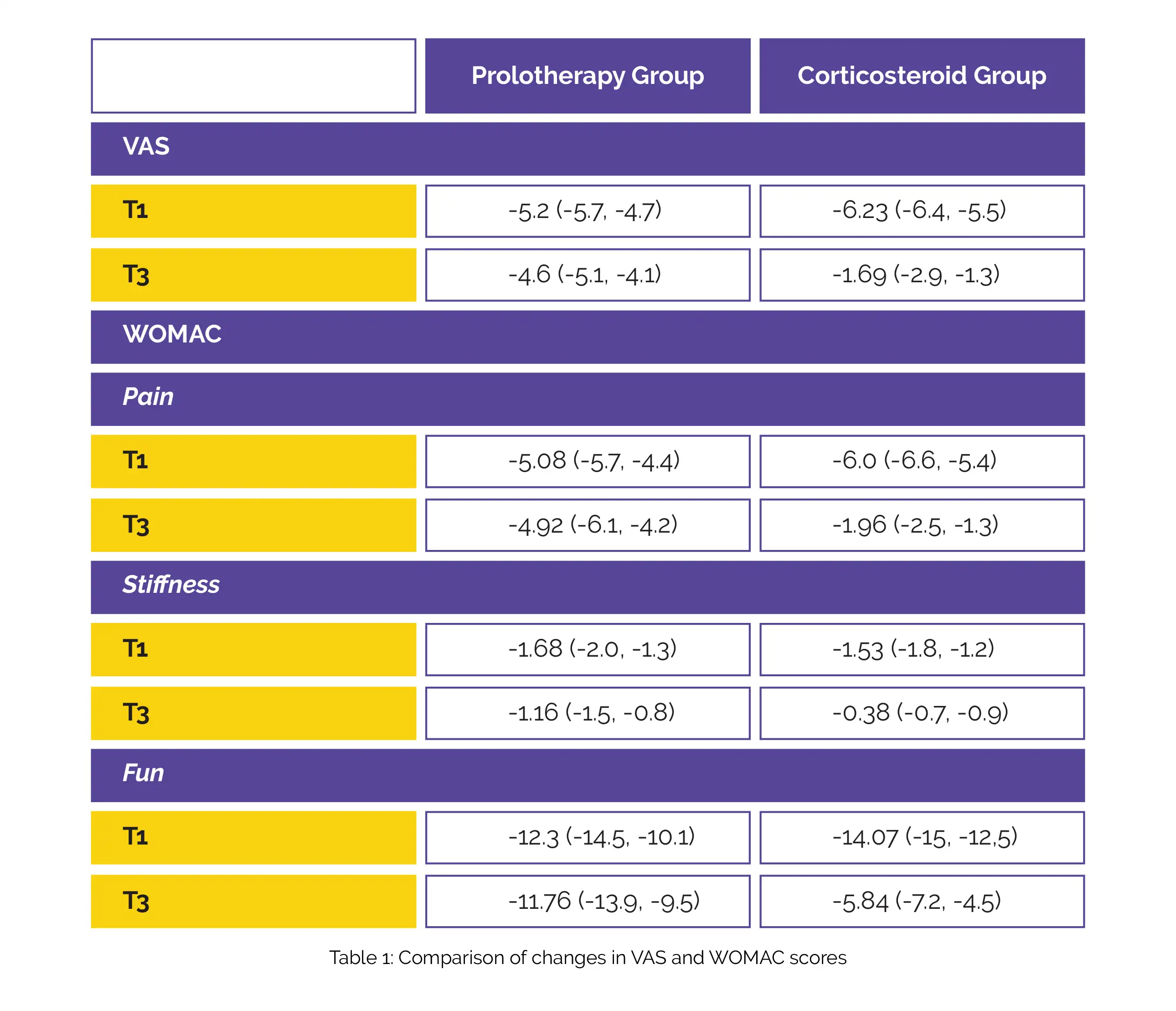
Individuals having knee osteoarthritis can benefit from both corticosteroid and Dextrose prolotherapy (combination of intra and extraarticular approaches) in terms of pain relief and functional improvement. The prolotherapy approach was less efficient in reducing pain in the short term than corticosteroids, but its benefits lasted longer, and in midterm tests (3 months), it outperformed corticosteroids.
Anesthesiology and Pain Medicine
Comparison of Dextrose Prolotherapy and Triamcinolone Intraarticular Injection on Pain and Function in Patients with Knee Osteoarthritis - A Randomized Clinical Trial
Masume Bayat et al.
Comments (0)