Categories
Change Password!
Reset Password!


The aim of this randomized, double-blind, placebo-controlled trial was to determine Sanjie Analgesic Capsule (SAC)'s effectiveness and safety for endometriosis-linked pain in Chinese women.
Sanjie Analgesic Capsule has good tolerability and can help to relieve chronic pain associated with endometriosis in women.
The aim of this randomized, double-blind, placebo-controlled trial was to determine Sanjie Analgesic Capsule (SAC)'s effectiveness and safety for endometriosis-linked pain in Chinese women.
This multicenter study in China involving 323 endometriosis patients was conducted between November 2013 and July 2017, with a 3:1 ratio between the SAC group (241 cases) and the placebo group (82 cases). Volunteers in the SAC or placebo groups received 1.6 g of SAC or placebo orally three times daily, starting on the first day of menstruation for three sequential cycles.
At 3 and 6 months, the key outcome ascertained was clinical response to dysmenorrhea, assessed utilizing a 10-point visual analogue scale (VAS). The secondary endpoints were pain score (assessed using VAS at 3 and 6 months) and pain recurrence rate (assessed at 6 months). Recording of adverse events (AEs) was done.
Out of 241 women in the SAC group and 82 in the placebo group, 217 (90.0%) and 71 (86.6%) completed the intervention, respectively. At 3 and 6 months, the overall response rate (ORR) for dysmenorrhea was remarkably more in women administered SAC compared to placebo recipients, as shown in Table 1:
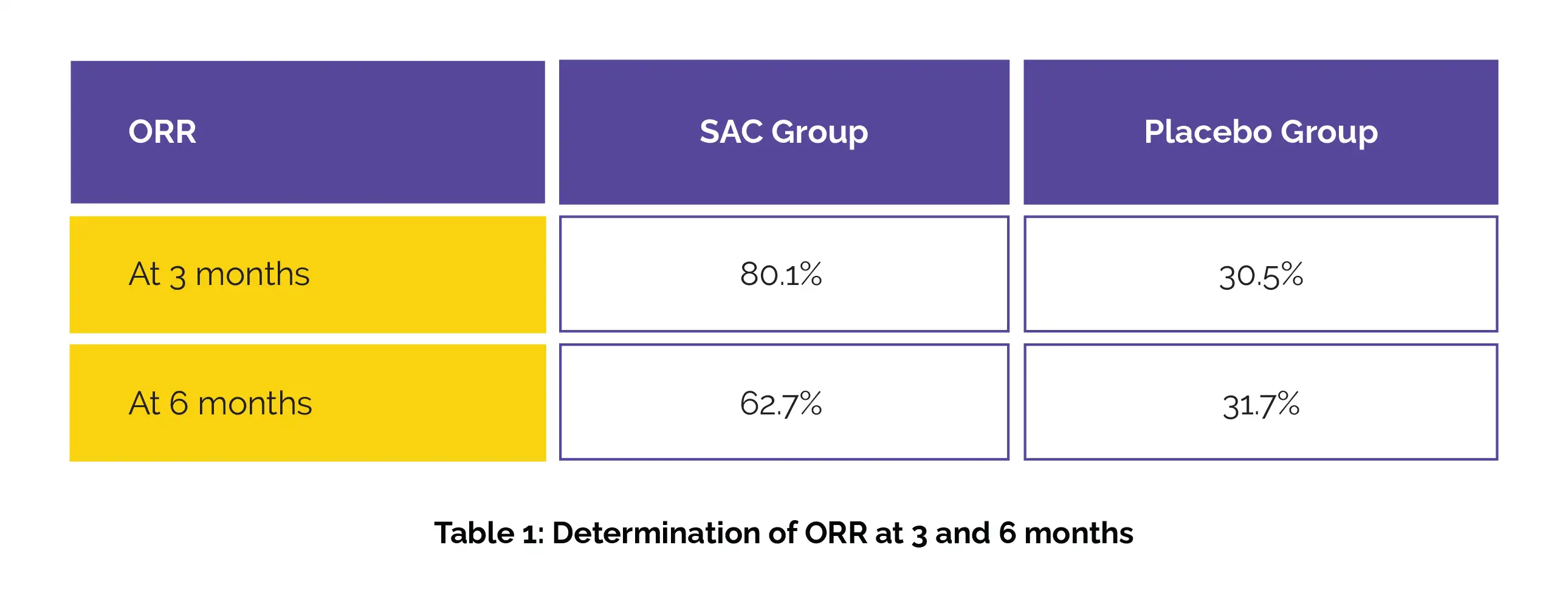
SAC also markedly improved chronic pelvic pain and defecation pain. Total AE incidence rates were 6.6% and 9.8%, respectively, with no vital difference between the two groups.
SAC is generally well-tolerated and can potentially alleviate dysmenorrhea in women experiencing endometriosis-related pain.
Chinese Journal of Integrative Medicine
Efficacy and Safety of Sanjie Analgesic Capsule in Patients with Endometriosis-Associated Pain: A Multicenter, 3:1 Randomized, Double-Blind, Placebo-Controlled Trial
Jin-Hua Leng et al.
Comments (0)